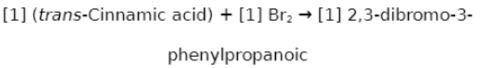Chemistry, 22.02.2020 02:06 jennyferluna0216
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and 2.30 mL of glacial acetic acid. After the reaction and workup, the student ended up with 0.2223 g of brominated product. Calculate the student\'s theoretical and percent yields.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and...
Questions

History, 15.02.2021 14:50

Physics, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50




Mathematics, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50

Chemistry, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50


Mathematics, 15.02.2021 14:50

SAT, 15.02.2021 14:50

History, 15.02.2021 14:50

Mathematics, 15.02.2021 14:50

Computers and Technology, 15.02.2021 14:50