Chemistry, 22.02.2020 04:36 sarahgarza5440
What is the relative atomic mass of a hypothetical element that consists of the following isotopes in the indicated natural abundances? Isotope - Isotopic mass (amuamu) - Relative abundance (%%) 1 - 78.9 - 12.6 2 - 80.9 - 13.9 3 - 82.9 - 73.5

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
What is the relative atomic mass of a hypothetical element that consists of the following isotopes i...
Questions



History, 12.07.2019 03:30

Health, 12.07.2019 03:30


Business, 12.07.2019 03:30




Mathematics, 12.07.2019 03:30

Biology, 12.07.2019 03:30



History, 12.07.2019 03:30

History, 12.07.2019 03:30


Mathematics, 12.07.2019 03:30

Chemistry, 12.07.2019 03:30

History, 12.07.2019 03:30

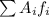
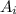 is the atomic mass of each isotope and
is the atomic mass of each isotope and 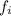 the relative frequency. Therefore, we find:
the relative frequency. Therefore, we find: