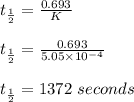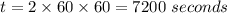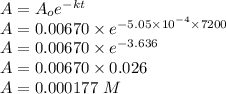Chemistry, 22.02.2020 04:59 francisebell2p698f2
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene gas. At a certain temperature, the rate constant for this reaction is 5.05 × 10 − 4 s − 1 . Calculate the half-life of cyclopropane at this temperature. t 1 / 2 = s Given an initial cyclopropane concentration of 0.00670 M , calculate the concentration of cyclopropane that remains after 2.00 hours. concentration.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene g...
Questions

Mathematics, 14.01.2021 19:10

English, 14.01.2021 19:10


Arts, 14.01.2021 19:10

Biology, 14.01.2021 19:10





Mathematics, 14.01.2021 19:10




Social Studies, 14.01.2021 19:10





Mathematics, 14.01.2021 19:10

English, 14.01.2021 19:10

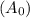 of was
of was 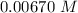 .
.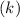 is
is 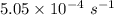
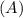 after 2 hours.
after 2 hours.