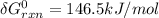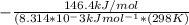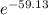Chemistry, 22.02.2020 05:26 maryam2863
Use data from appendix iib in the textbook to calculate the equilibrium constants at 25 degrees Celsius for each of the following reactions:
a. 2 CO (g) O2(g) <-> 2CO2 (g)
b. 2H2S (g) <-> 2H2 (g) S2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

You know the right answer?
Use data from appendix iib in the textbook to calculate the equilibrium constants at 25 degrees Cels...
Questions




Mathematics, 28.10.2020 03:40


Mathematics, 28.10.2020 03:40

Mathematics, 28.10.2020 03:40

Chemistry, 28.10.2020 03:40

Health, 28.10.2020 03:40


Biology, 28.10.2020 03:40

History, 28.10.2020 03:40

Mathematics, 28.10.2020 03:40

History, 28.10.2020 03:40

Advanced Placement (AP), 28.10.2020 03:40

English, 28.10.2020 03:40


History, 28.10.2020 03:40

English, 28.10.2020 03:40

Mathematics, 28.10.2020 03:40

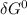 = -RT㏑K
= -RT㏑K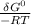 ----------------- equation (1)
----------------- equation (1)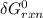 = ???
= ???![\delta G^0_{rxn} =[2*\delta G^0_{f(co_2)}-(2*\deltaG^0_{f(co)}+1*\delta G^0_{f(o_2)})]kJ/mol](/tpl/images/0520/1906/6c481.png)
![\delta G^0_{rxn} =[2*394.4-(2*137.2+1*0)]kJ/mol](/tpl/images/0520/1906/8f15d.png)
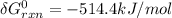
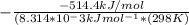
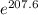
![\delta G^0_{rxn} =[(2*\delta G^0_{f(H_2)}+1*\delta G^0_{f(S_2)})-2*\delta G^0_{f(H_2S)})]kJ/mol](/tpl/images/0520/1906/7404c.png)
![\delta G^0_{rxn} =[(2*0)+(1*79.7)-(2*-33.4})]kJ/mol](/tpl/images/0520/1906/4c99d.png)