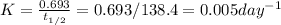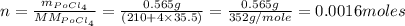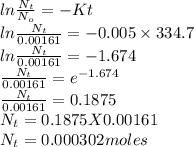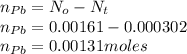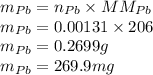Chemistry, 22.02.2020 06:17 antbanks3050
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
210 206 4
84 Po > 82Pb + 2 He
If the half-life, t1/2, of 210Po is 138.4 days, calculate the mass of 206Pb that can be produced from a 565.0-mg sample of polonium(IV) chloride, PoCl4, that is left untouched for 334.7 days. Assume that the only polonium isotope present in the sample is the 210Po isotope. The isotopic molar masses of 210Po is 209.98 g/mol and 206Pb is 205.97 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Polonium-210, 210Po, decays to lead-206, 206Pb, by alpha emission according to the equation
Questions

Mathematics, 08.07.2021 20:20

Mathematics, 08.07.2021 20:20




Arts, 08.07.2021 20:20











Biology, 08.07.2021 20:20


Computers and Technology, 08.07.2021 20:20

Advanced Placement (AP), 08.07.2021 20:20