
Chemistry, 22.02.2020 09:38 XxKaitlynnxX
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be produced from a reaction 700.0 kg of aluminum oxide to produce Al? (assume that the reaction is Al2O3 + H2=Al+H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3
You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions




Mathematics, 06.12.2019 01:31

Biology, 06.12.2019 01:31


Mathematics, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31




Mathematics, 06.12.2019 01:31

History, 06.12.2019 01:31

Chemistry, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31



Mathematics, 06.12.2019 01:31

Mathematics, 06.12.2019 01:31

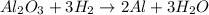
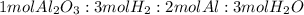
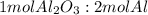 ; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.
; thus 6,865.4 mol of aluminium oxide produce 2 × 6,865.4 mol of aluminum = 13,730.8 mol of aluminium.

