Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
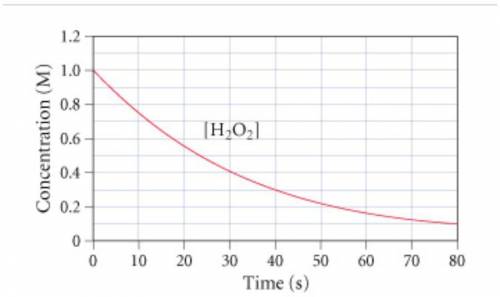
The graph (Figure 1...

Chemistry, 22.02.2020 20:03 beanokelley
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L , what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Questions

History, 02.03.2020 03:40

Computers and Technology, 02.03.2020 03:40

Health, 02.03.2020 03:40

History, 02.03.2020 03:40

Computers and Technology, 02.03.2020 03:41


Mathematics, 02.03.2020 03:41


Mathematics, 02.03.2020 03:41



Mathematics, 02.03.2020 03:42




Mathematics, 02.03.2020 03:42



Mathematics, 02.03.2020 03:43



