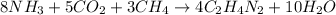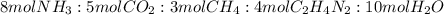Chemistry, 22.02.2020 22:20 Scourge927
One of the intermediates in the synthesis of glycine from ammonia, carbon dioxide, and methane is aminoacetonitrile, C2H4N2.
How much C2H4N2 could be expected from the reaction of 14.9 g CO2, 2.11 g NH3, and 1.75 g CH4?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
One of the intermediates in the synthesis of glycine from ammonia, carbon dioxide, and methane is am...
Questions

Mathematics, 26.07.2019 01:00



Mathematics, 26.07.2019 01:00

Computers and Technology, 26.07.2019 01:00




Mathematics, 26.07.2019 01:00


Mathematics, 26.07.2019 01:00