Chemistry, 23.02.2020 00:22 JamierW2005
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element.
A. Protons = 50
Neutrons = 118
Protons = 46
B. Protons = 68
Neutrons = 46
Electrons = 54
C. Protons = 50
Neutrons = 68
Electrons = 46
D. Protons = 118
Neutrons = 50
Electrons = 46
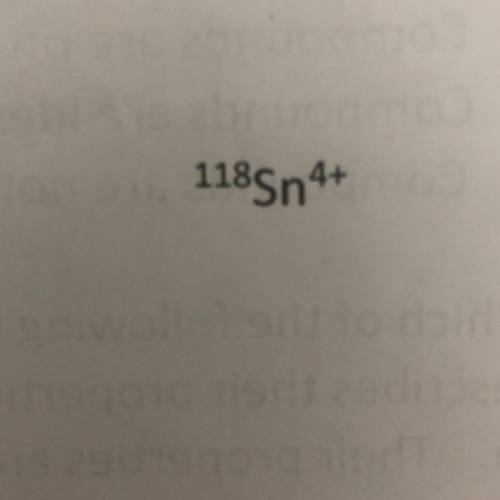

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element...
Questions




Mathematics, 12.04.2021 14:00

History, 12.04.2021 14:00


Mathematics, 12.04.2021 14:00

Social Studies, 12.04.2021 14:00

Mathematics, 12.04.2021 14:00

Health, 12.04.2021 14:00

Geography, 12.04.2021 14:00

Spanish, 12.04.2021 14:00


Mathematics, 12.04.2021 14:00

Computers and Technology, 12.04.2021 14:00

Geography, 12.04.2021 14:00