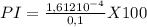Chemistry, 23.02.2020 05:56 isaacbryan2416
A certain weak acid, HA, has a Ka value of 2.6×10−7. Calculate the percent ionization of HA in a 0.10 M solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
A certain weak acid, HA, has a Ka value of 2.6×10−7. Calculate the percent ionization of HA in a 0.1...
Questions






Social Studies, 08.10.2019 02:30







Biology, 08.10.2019 02:30



Social Studies, 08.10.2019 02:30


Chemistry, 08.10.2019 02:30

Mathematics, 08.10.2019 02:30

Mathematics, 08.10.2019 02:30

![PI=\frac{[A-]}{[HA]} x100](/tpl/images/0520/5455/031dd.png)
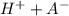
![Ka=\frac{[H+][A-]}{[HA]}\\ \\](/tpl/images/0520/5455/b886f.png)
![Ka=\frac{[H+]^{2} }{[HA]} \\\\](/tpl/images/0520/5455/c597e.png)
![[H+]=\sqrt[2]{Ka.[HA]} \\\\](/tpl/images/0520/5455/88b1b.png)
![[H+] =\sqrt{(2,610^{-7} )(0,1)} = 1,61210^{-4}](/tpl/images/0520/5455/d2b22.png)