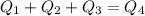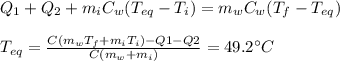Chemistry, 23.02.2020 09:44 dreawongdga
Calculate the final temperature of a mixture of 200.0 g of ice initially at -20.50 °C and 319.0 g of water initially at 91.50 °C.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
Calculate the final temperature of a mixture of 200.0 g of ice initially at -20.50 °C and 319.0 g of...
Questions



History, 19.09.2021 21:40

Mathematics, 19.09.2021 21:40


Spanish, 19.09.2021 21:40


English, 19.09.2021 21:40

English, 19.09.2021 21:40

Mathematics, 19.09.2021 21:40


Geography, 19.09.2021 21:40



Chemistry, 19.09.2021 21:40


Mathematics, 19.09.2021 21:40

Advanced Placement (AP), 19.09.2021 21:40


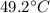
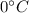 ), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature.
), part is used to melt the ice, and the rest is used to increase the temperature of the ice (which is now melted) to the equilibrium temperature. 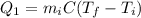
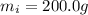 is the mass of the ice
is the mass of the ice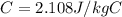 is the specific heat of ice
is the specific heat of ice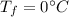 is the final temperature
is the final temperature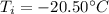 is the initial temperature
is the initial temperature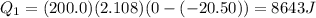
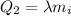
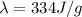 is the specific latent heat of ice
is the specific latent heat of ice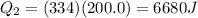
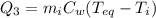
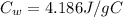 is the specific heat of water
is the specific heat of water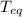 is the equilibrium temperature
is the equilibrium temperature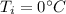 is the initial temperature of ice
is the initial temperature of ice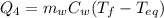
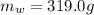 is the mass of the water
is the mass of the water is the initial temperature of the water
is the initial temperature of the water