WILL MARK BRAINLIEST FOR CORRECT ANSWER!
Methane burns in oxygen to produce carbon diox...

WILL MARK BRAINLIEST FOR CORRECT ANSWER!
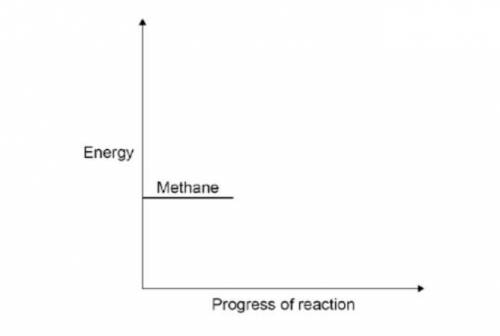
Methane burns in oxygen to produce carbon dioxide and water. The activation energy for the reaction is 2648 kJ / mol. The reaction give 818 kJ / mol. The reaction gives out 818 kJ / mol of energy. Complete the reaction profile. Draw arrows to represent:
the activation energy
the energy given out.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3


You know the right answer?
Questions


Social Studies, 20.03.2020 09:44

Mathematics, 20.03.2020 09:44





Mathematics, 20.03.2020 09:45












Social Studies, 20.03.2020 09:46




