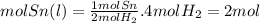At 1023 K and 1 atm, a 3.00 gram sample of Snoz(s) (gram-formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as
shown in the balanced equation below:
SnO2 (s) + 2H2(g) + Sn(l) + 2H2O(g)
Determine the number
of moles of sn(l)
produced when 4.0
Moles of H2(g) is completely consumed

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
At 1023 K and 1 atm, a 3.00 gram sample of Snoz(s) (gram-formula mass = 151 g/mol) reacts with hydro...
Questions

Chemistry, 27.10.2020 18:40





Mathematics, 27.10.2020 18:40

Geography, 27.10.2020 18:40




History, 27.10.2020 18:40


Mathematics, 27.10.2020 18:40






Mathematics, 27.10.2020 18:40

History, 27.10.2020 18:40

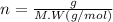
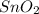 (s) + 2
(s) + 2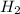 (g) ⇒ Sn(l) + 2
(g) ⇒ Sn(l) + 2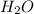 (g)
(g)