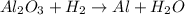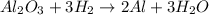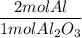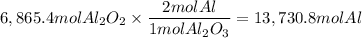Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2


Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions

Mathematics, 02.02.2021 08:00


Mathematics, 02.02.2021 08:00


Mathematics, 02.02.2021 08:00



Arts, 02.02.2021 08:00

English, 02.02.2021 08:00


English, 02.02.2021 08:00

Mathematics, 02.02.2021 08:00


Mathematics, 02.02.2021 08:00




English, 02.02.2021 08:00

Geography, 02.02.2021 08:00