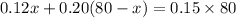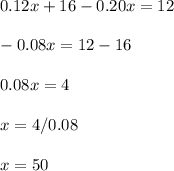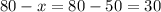Chemistry, 24.02.2020 16:59 whitneyt2013
A pharmacist has a 12% solution of boric acid and a 20% solution of boric acid. How much of each must he use to make 80 grams of a 15% boric acid solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
A pharmacist has a 12% solution of boric acid and a 20% solution of boric acid. How much of each mus...
Questions

English, 23.03.2021 02:20

Mathematics, 23.03.2021 02:20



Mathematics, 23.03.2021 02:20


English, 23.03.2021 02:20


Mathematics, 23.03.2021 02:20

Biology, 23.03.2021 02:20


Computers and Technology, 23.03.2021 02:20





English, 23.03.2021 02:20



Chemistry, 23.03.2021 02:20