Chemistry, 24.02.2020 18:28 ninja12302
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 320.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions

Mathematics, 09.12.2021 22:20

Mathematics, 09.12.2021 22:20

Mathematics, 09.12.2021 22:20



History, 09.12.2021 22:20

Mathematics, 09.12.2021 22:20



English, 09.12.2021 22:20

Arts, 09.12.2021 22:20

History, 09.12.2021 22:20


Mathematics, 09.12.2021 22:20






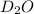 needed is 0.96 grams
needed is 0.96 grams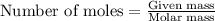 .....(1)
.....(1)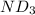 :
: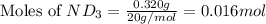
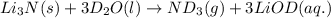
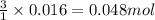 of
of