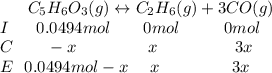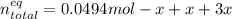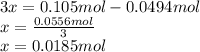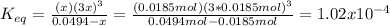Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g sample of pure C5H6O3(g) was placed in an evacuated 2.50 L flask and heated to 200.ºC. At equilibrium, the pressure in the flask was 1.63 atm. Calculate K for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g...
Questions

Geography, 01.08.2019 17:50

Mathematics, 01.08.2019 17:50

History, 01.08.2019 17:50

Social Studies, 01.08.2019 17:50

Mathematics, 01.08.2019 17:50

English, 01.08.2019 17:50



Chemistry, 01.08.2019 17:50



History, 01.08.2019 17:50


History, 01.08.2019 17:50


History, 01.08.2019 17:50

English, 01.08.2019 17:50

Chemistry, 01.08.2019 17:50

English, 01.08.2019 17:50

Mathematics, 01.08.2019 17:50

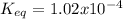
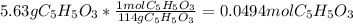
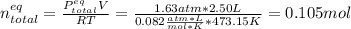
 ", which yields to:
", which yields to: