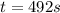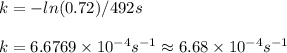Chemistry, 24.02.2020 18:41 kprincess16r
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the presence of an excess of argon. Measurement at 500. K reveals that in 492 seconds, the concentration of CH3NC has decreased to 72% of its original value. Calculate the rate constant (k) of the reaction at 500. K.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the pre...
Questions

Computers and Technology, 17.12.2020 14:50



Mathematics, 17.12.2020 15:00

Mathematics, 17.12.2020 15:00

History, 17.12.2020 15:00


Computers and Technology, 17.12.2020 15:00


Mathematics, 17.12.2020 15:00

English, 17.12.2020 15:00



Mathematics, 17.12.2020 15:00

History, 17.12.2020 15:00

Social Studies, 17.12.2020 15:00


History, 17.12.2020 15:00

Computers and Technology, 17.12.2020 15:00

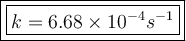
![\dfrac{d[CH_3NC]}{[CH_3NC]}=-kt](/tpl/images/0521/4081/74427.png)
![[CH_3NC]=[CH_3NC]_0e^{-kt}](/tpl/images/0521/4081/d933f.png)
![\dfrac{CH_3NC]}{[CH_3NC]_0}=0.72](/tpl/images/0521/4081/eda95.png)