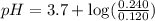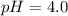Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Calculate the pH of a solution that is 0.240 M in sodium formate (HCOONa) and 0.120 M in formic acid...
Questions

Mathematics, 31.12.2019 09:31


History, 31.12.2019 09:31


Mathematics, 31.12.2019 09:31

Biology, 31.12.2019 09:31

Social Studies, 31.12.2019 09:31

Biology, 31.12.2019 09:31

English, 31.12.2019 09:31




Mathematics, 31.12.2019 09:31


Mathematics, 31.12.2019 09:31


Mathematics, 31.12.2019 09:31

History, 31.12.2019 09:31


English, 31.12.2019 09:31

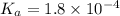
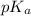 .
.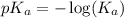
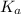 in this expression, we get:
in this expression, we get: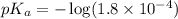
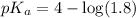
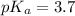
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0521/4495/e961a.png)