
Chemistry, 24.02.2020 19:29 hannahkharel2
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life f...
Questions


Mathematics, 09.02.2021 17:00


Mathematics, 09.02.2021 17:00

English, 09.02.2021 17:00

Mathematics, 09.02.2021 17:00

Biology, 09.02.2021 17:00



Computers and Technology, 09.02.2021 17:00



Biology, 09.02.2021 17:00

Health, 09.02.2021 17:00

Mathematics, 09.02.2021 17:00

Health, 09.02.2021 17:00


English, 09.02.2021 17:00

English, 09.02.2021 17:00

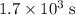 .
. 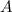 .) The half-life
.) The half-life 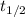 of that first-order reaction would be the time it takes for
of that first-order reaction would be the time it takes for ![[A]](/tpl/images/0521/5010/6aa06.png) (concentration of
(concentration of 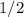 its initial value.
its initial value.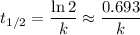 ,
, 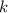 is the rate constant of this reaction.
is the rate constant of this reaction.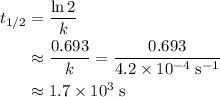 .
.

