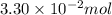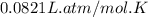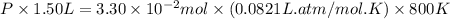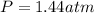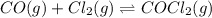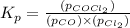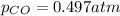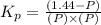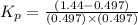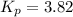Chemistry, 24.02.2020 22:28 pattydixon6
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K, and at equilibrium the pressure of CO was found to be 0.497 atm. Calculate the equilibrium constant, Kp, for the reaction: CO(g) Cl2(g) COCl2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

You know the right answer?
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K...
Questions


Mathematics, 02.11.2020 19:50


Engineering, 02.11.2020 19:50

Mathematics, 02.11.2020 19:50

Business, 02.11.2020 19:50








Mathematics, 02.11.2020 19:50

Biology, 02.11.2020 19:50

History, 02.11.2020 19:50

Spanish, 02.11.2020 19:50

History, 02.11.2020 19:50


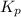 for the reaction is, 3.82
for the reaction is, 3.82