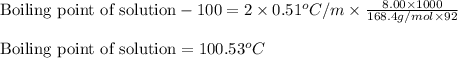Chemistry, 24.02.2020 23:27 ashleybarrera2000
An aqueous CsCl solution is 8.00 wt% CsCl and has a density of 1.0643 g/mL at 20°C. What is the boiling point of this solution? Kb = 0.51°C/m for water. Enter your answer with 2 decimal places and no units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
An aqueous CsCl solution is 8.00 wt% CsCl and has a density of 1.0643 g/mL at 20°C. What is the boil...
Questions




Geography, 04.02.2020 09:53



Biology, 04.02.2020 09:53

Chemistry, 04.02.2020 09:53





History, 04.02.2020 09:53


Mathematics, 04.02.2020 09:53

Social Studies, 04.02.2020 09:53

Mathematics, 04.02.2020 09:53

History, 04.02.2020 09:53

Mathematics, 04.02.2020 09:53

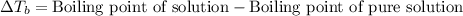
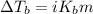
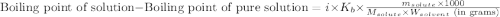
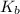 = molal boiling point elevation constant = 0.51°C/m
= molal boiling point elevation constant = 0.51°C/m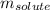 = Given mass of solute (CsCl) = 8.00 g
= Given mass of solute (CsCl) = 8.00 g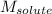 = Molar mass of solute (CsCl) = 168.4 g/mol
= Molar mass of solute (CsCl) = 168.4 g/mol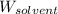 = Mass of solvent (water) = 92 g
= Mass of solvent (water) = 92 g