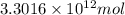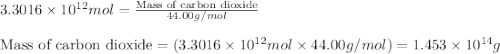Chemistry, 24.02.2020 23:39 mariahrpoulin9630
Assuming gasoline is isooctane, with a density of g/mL, what is the theoretical yield of carbon dioxide produced by the combustion of gal of gasoline (the approximate annual consumption of gasoline in the United States)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Assuming gasoline is isooctane, with a density of g/mL, what is the theoretical yield of carbon diox...
Questions

English, 25.03.2020 10:07



Mathematics, 25.03.2020 10:07


English, 25.03.2020 10:07

English, 25.03.2020 10:07

Mathematics, 25.03.2020 10:08

Mathematics, 25.03.2020 10:08





Chemistry, 25.03.2020 10:10

Mathematics, 25.03.2020 10:10

Chemistry, 25.03.2020 10:11

English, 25.03.2020 10:11

Mathematics, 25.03.2020 10:11

Mathematics, 25.03.2020 10:11

Mathematics, 25.03.2020 10:11

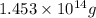
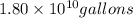
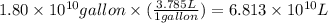
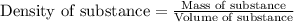
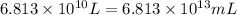 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)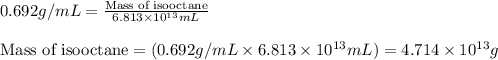
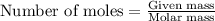 .....(1)
.....(1)
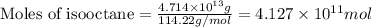
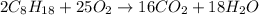
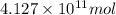 of isooctane will produce =
of isooctane will produce = 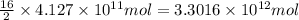 of carbon dioxide
of carbon dioxide