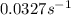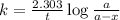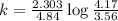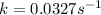Chemistry, 24.02.2020 23:41 davisbrittany5784
Consider the following first-order reaction: A → B. The concentration of A at the start of the reaction is 4.17 M and after 4.84 s is 3.56 M. (a) Using the integrated rate law for a first-order reaction, calculate the value of the rate constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

You know the right answer?
Consider the following first-order reaction: A → B. The concentration of A at the start of the react...
Questions



Mathematics, 12.09.2021 04:30


Mathematics, 12.09.2021 04:30

Mathematics, 12.09.2021 04:30


Biology, 12.09.2021 04:30


Business, 12.09.2021 04:30






Business, 12.09.2021 04:30


Mathematics, 12.09.2021 04:30

Mathematics, 12.09.2021 04:30

Mathematics, 12.09.2021 04:30