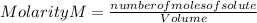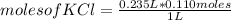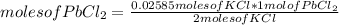Chemistry, 25.02.2020 00:04 andreanb8083
According to the following reaction, what mass of PbCl2 can form from 235 mL of 0.110 M KCl solution? Assume that there is excess Pb(NO3)2. 2 KCl(aq) + Pb(NO3)2(aq) → PbCl2(s) + 2 KNO3(aq)

Answers: 2


Another question on Chemistry




Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
According to the following reaction, what mass of PbCl2 can form from 235 mL of 0.110 M KCl solution...
Questions

History, 27.08.2021 04:40


Mathematics, 27.08.2021 04:40


Mathematics, 27.08.2021 04:40


Chemistry, 27.08.2021 04:40





Mathematics, 27.08.2021 04:40

Mathematics, 27.08.2021 04:40

Biology, 27.08.2021 04:40



History, 27.08.2021 04:40