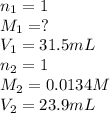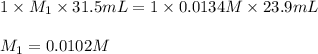Chemistry, 25.02.2020 00:43 KillerSteamcar
A 31.5mL aliquot of HNO3 (aq) of unknown concentration wastitrated with 0.0134 M NaOH (aq). It took 23.9 mL of the base toreach the endpoint of the titration. The concentration (M) of theacid was .I forget how to do titration problems and cannot seem to find anysimilar examples in my text. Is there a specific formula you usefor these?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1

Chemistry, 23.06.2019 17:00
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
A 31.5mL aliquot of HNO3 (aq) of unknown concentration wastitrated with 0.0134 M NaOH (aq). It took...
Questions



Mathematics, 23.04.2020 23:33




Mathematics, 23.04.2020 23:33


Computers and Technology, 23.04.2020 23:33

History, 23.04.2020 23:33

Spanish, 23.04.2020 23:33

Social Studies, 23.04.2020 23:33

Mathematics, 23.04.2020 23:33

Physics, 23.04.2020 23:33

English, 23.04.2020 23:33

History, 23.04.2020 23:33

Mathematics, 23.04.2020 23:33

English, 23.04.2020 23:33


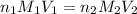
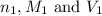 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 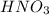
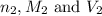 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.