Chemistry, 25.02.2020 00:50 SucMaDongShan
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mol for the reaction. Do not include units. Report your answer to 3 significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2


Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mo...
Questions


Social Studies, 21.09.2019 17:30


Physics, 21.09.2019 17:30

Mathematics, 21.09.2019 17:30


Biology, 21.09.2019 17:30



Chemistry, 21.09.2019 17:30

Mathematics, 21.09.2019 17:30


Mathematics, 21.09.2019 17:30






History, 21.09.2019 17:30

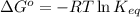
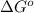 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?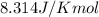
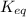 = equilibrium constant = 581
= equilibrium constant = 581