
Chemistry, 25.02.2020 00:49 ninaaforever
Please explain how the following two combustion (burning) reactions appear to disobey the law of conservation of Mass: A When a diamond is burned, the mass that remains is zero. B. When a piece of wood is burned, the leftover ashes weight less than the wood. Upon further investigation, it is noted that CO2 is vented off from both reactions. With this additional information, state how you can prove the diamond is made of pure carbon ans yet the wood is not.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1


You know the right answer?
Please explain how the following two combustion (burning) reactions appear to disobey the law of con...
Questions




Social Studies, 22.06.2021 14:00

Mathematics, 22.06.2021 14:10

History, 22.06.2021 14:10

Social Studies, 22.06.2021 14:10


Business, 22.06.2021 14:10

Mathematics, 22.06.2021 14:10

Business, 22.06.2021 14:10

Mathematics, 22.06.2021 14:20


English, 22.06.2021 14:20

English, 22.06.2021 14:20

Mathematics, 22.06.2021 14:20

English, 22.06.2021 14:20

Mathematics, 22.06.2021 14:20

Biology, 22.06.2021 14:20

Chemistry, 22.06.2021 14:20

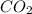 , water, and light energy which proves that the mass after the reaction is less.Burning of allotropic forms of carbon releases carbon dioxide,water,light and heat energy.C(s) + O(g) =
, water, and light energy which proves that the mass after the reaction is less.Burning of allotropic forms of carbon releases carbon dioxide,water,light and heat energy.C(s) + O(g) = 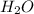 + light energy.Diamond is strongly covalent bonded and can be combustible at very high temperatures.When a diamond is burned the mass remains zero as diamond is formed by pure carbon atom and gives off carbon dioxide and water.Therefore, wood, as well as the diamond, disobey the law of conservation of mass.
+ light energy.Diamond is strongly covalent bonded and can be combustible at very high temperatures.When a diamond is burned the mass remains zero as diamond is formed by pure carbon atom and gives off carbon dioxide and water.Therefore, wood, as well as the diamond, disobey the law of conservation of mass.

