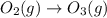Ozone gas is a form of elemental oxygen containing molecules with three oxygen atoms, O3. Ozone is produced from atmospheric oxygen gas O2, by the high energy outbursts found in lightening storms. Write the unbalanced equation for the formation of ozone gas from oxygen gas.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Ozone gas is a form of elemental oxygen containing molecules with three oxygen atoms, O3. Ozone is p...
Questions

Mathematics, 24.09.2019 14:20


Biology, 24.09.2019 14:20



Biology, 24.09.2019 14:20



History, 24.09.2019 14:20


Mathematics, 24.09.2019 14:20

Mathematics, 24.09.2019 14:20


Computers and Technology, 24.09.2019 14:20

History, 24.09.2019 14:20

Mathematics, 24.09.2019 14:20


Chemistry, 24.09.2019 14:20