
Chemistry, 25.02.2020 01:47 kitttimothy55
Consider the following reaction at equilibrium: 2 NH3(g) ⇄ N2(g) + 3 H2(g) What does Le Chatelier's principle predict will happen when adding N2 (g) to the system at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Consider the following reaction at equilibrium: 2 NH3(g) ⇄ N2(g) + 3 H2(g) What does Le Chatelier's...
Questions

Business, 13.04.2020 05:48

Mathematics, 13.04.2020 05:48

Mathematics, 13.04.2020 05:50

Biology, 13.04.2020 05:50


Arts, 13.04.2020 06:06


Social Studies, 13.04.2020 06:06




Physics, 13.04.2020 06:07

Mathematics, 13.04.2020 06:07

History, 13.04.2020 06:07

Social Studies, 13.04.2020 06:07

Mathematics, 13.04.2020 06:07

Mathematics, 13.04.2020 06:07

Mathematics, 13.04.2020 06:07


Mathematics, 13.04.2020 06:07

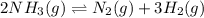
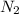 then the concentration of
then the concentration of 

