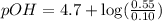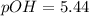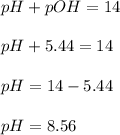Chemistry, 25.02.2020 02:57 fangirl2837
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that is 0.10M in aqueous ammonia and 0.55 M in ammonium nitrate. assume no volume change. (The Kb for NH3 =1.8 * 10-5 )

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that i...
Questions





English, 05.07.2021 16:30



Mathematics, 05.07.2021 16:40



Social Studies, 05.07.2021 16:40

Physics, 05.07.2021 16:40


Spanish, 05.07.2021 16:40


Mathematics, 05.07.2021 16:40

Mathematics, 05.07.2021 16:40

Mathematics, 05.07.2021 16:40


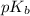 .
.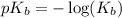
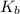 in this expression, we get:
in this expression, we get: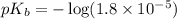
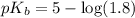
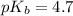
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0522/5805/ac570.png)