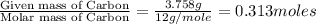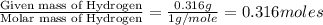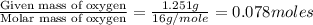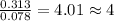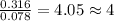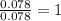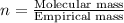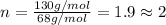Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3

Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1
You know the right answer?
A 5.325g sample of methyl benzoate, a compound in perfumes , was found to contain 3.758 g of carbon,...
Questions



Spanish, 18.10.2019 09:30

Mathematics, 18.10.2019 09:30

Physics, 18.10.2019 09:30




Mathematics, 18.10.2019 09:30

English, 18.10.2019 09:30

Mathematics, 18.10.2019 09:30


Mathematics, 18.10.2019 09:30


Mathematics, 18.10.2019 09:30

Social Studies, 18.10.2019 09:30

Computers and Technology, 18.10.2019 09:30



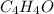 and
and 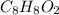 respectively
respectively