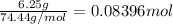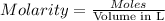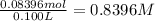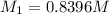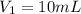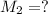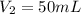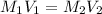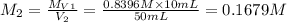Chemistry, 25.02.2020 04:01 swelch2010
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumetric flask using a volumetric pipet. The solution was then diluted to the mark with distilled water. Assuming that the concentration of a stock sodium hypochlorite is 6.25% (w/v), calculate the molarity of the diluted sodium hypochlorite solution. Molar mass of sodium hypochlorite

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1



You know the right answer?
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumet...
Questions

Mathematics, 18.10.2020 14:01

Physics, 18.10.2020 14:01

English, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Biology, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01





Health, 18.10.2020 14:01


History, 18.10.2020 14:01





Mathematics, 18.10.2020 14:01