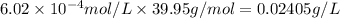Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
What is the solubility of argon (in units of grams per liter) in water at 25 °C, when the Ar gas ove...
Questions


Mathematics, 10.12.2019 13:31

History, 10.12.2019 13:31

English, 10.12.2019 13:31


Mathematics, 10.12.2019 13:31

Chemistry, 10.12.2019 13:31



Social Studies, 10.12.2019 13:31

Chemistry, 10.12.2019 13:31





Biology, 10.12.2019 13:31


History, 10.12.2019 13:31

Biology, 10.12.2019 13:31


 = Henry's constant =
= Henry's constant = 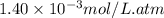
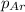 = partial pressure of carbonated drink = 0.51atm
= partial pressure of carbonated drink = 0.51atm