
Chemistry, 25.02.2020 04:36 dogeking12
A first-order reaction (A → B) has a half-life of 25 minutes. If the initial concentration of A is 0.900 M, what is the concentration of B after 50 minutes?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
A first-order reaction (A → B) has a half-life of 25 minutes. If the initial concentration of A is 0...
Questions

Mathematics, 20.09.2020 02:01

Computers and Technology, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Computers and Technology, 20.09.2020 02:01

History, 20.09.2020 02:01

Social Studies, 20.09.2020 02:01


History, 20.09.2020 02:01

Chemistry, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01



History, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Social Studies, 20.09.2020 02:01

French, 20.09.2020 02:01

English, 20.09.2020 02:01

English, 20.09.2020 02:01


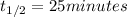
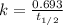
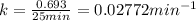
![[A]_o=0.900 M](/tpl/images/0522/8207/2af3f.png)
![[A]=?](/tpl/images/0522/8207/8be21.png)
![[A]=[A]_o\times e^{-kt}](/tpl/images/0522/8207/9e0b6.png)
![[A]=0.900 M\times e^{-0.02772 min^{-1}\times 50 minutes}](/tpl/images/0522/8207/3acb3.png)


