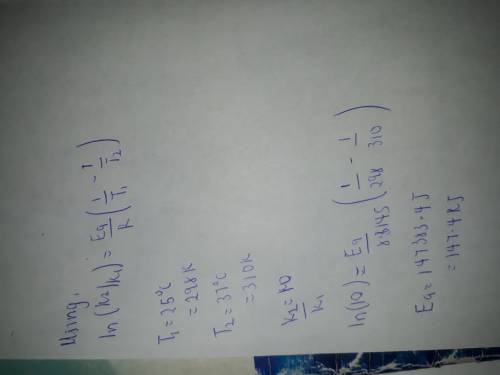Chemistry, 25.02.2020 04:33 floressavanna15
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rate constant of a reaction by ten-fold, what is the value for the activation energy of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rat...
Questions




Mathematics, 06.05.2020 00:41


Biology, 06.05.2020 00:41


Mathematics, 06.05.2020 00:41

Health, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41

Chemistry, 06.05.2020 00:41

Mathematics, 06.05.2020 00:41

History, 06.05.2020 00:41

Chemistry, 06.05.2020 00:41

Law, 06.05.2020 00:41


Mathematics, 06.05.2020 00:41