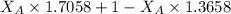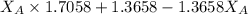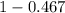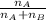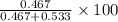A student was trying to determine the mole percent of A in a mixture of A and B using refractive index. If their mixture has a refractive index of 1.5248 and pure A and pure B each had refractive indices of 1.7058 and 1.3658, respectively, what was the mole percent of A in their mixture. Type your numerical answer rounded to the 3rd decimal place (i. e. 45.982 or 9.550, etc) without a percent sign.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
A student was trying to determine the mole percent of A in a mixture of A and B using refractive ind...
Questions

History, 30.10.2019 19:31

Chemistry, 30.10.2019 19:31


Biology, 30.10.2019 19:31

Biology, 30.10.2019 19:31






History, 30.10.2019 19:31

Biology, 30.10.2019 19:31


History, 30.10.2019 19:31




Mathematics, 30.10.2019 19:31


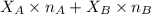
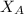 = mole fraction of A
= mole fraction of A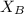 = mole fraction of B
= mole fraction of B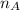 = refractive index of A
= refractive index of A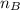 = refractive index of B
= refractive index of B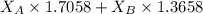 ........ (1)
........ (1)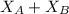 = 1
= 1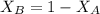 ......... (2)
......... (2)