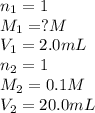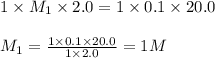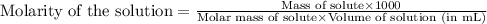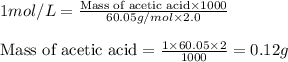Chemistry, 25.02.2020 05:12 rosetoheart2
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titrate it to its endpoint with 20.0 mL NaOH (0.1 M). What mass of acetic acid was dissolved in the 2.0 mL of solution used?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titra...
Questions

Biology, 03.07.2019 17:00

Social Studies, 03.07.2019 17:00


Business, 03.07.2019 17:00


History, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00







Mathematics, 03.07.2019 17:00


Mathematics, 03.07.2019 17:00


Mathematics, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00

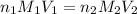
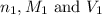 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 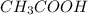
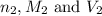 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.