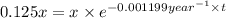Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
A decomposition reaction has a half life of 578 years. (a) What is the rate constant for this reacti...
Questions

Mathematics, 02.04.2021 20:30


Advanced Placement (AP), 02.04.2021 20:30

Mathematics, 02.04.2021 20:30

Mathematics, 02.04.2021 20:30

Mathematics, 02.04.2021 20:30



Mathematics, 02.04.2021 20:30


Mathematics, 02.04.2021 20:30

English, 02.04.2021 20:30

Mathematics, 02.04.2021 20:30

Mathematics, 02.04.2021 20:30

Mathematics, 02.04.2021 20:30



Mathematics, 02.04.2021 20:30


History, 02.04.2021 20:30

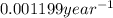 is the rate constant for this reaction.
is the rate constant for this reaction.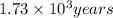 to concentration to reach 12.5% of its original value.
to concentration to reach 12.5% of its original value.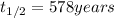
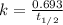
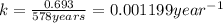
![[A_o]](/tpl/images/0522/9110/dc622.png) = x
= x![[A]](/tpl/images/0522/9110/6aa06.png) = 12.5% of x = 0.125x
= 12.5% of x = 0.125x![[A]=[A_o]\times e^{-kt}](/tpl/images/0522/9110/abdec.png)