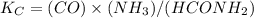Chemistry, 25.02.2020 06:13 BIKRAMlawati5544
Onsider the reaction, which takes place at a certain elevated temperature CO(g)+NH3(g)⇌HCONH2(g), Kc=0.860 If a reaction vessel initially contains only CO and NH3 at concentrations of 1.00 M and 2.00 M, respectively, what will the concentration of HCONH2 be at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

You know the right answer?
Onsider the reaction, which takes place at a certain elevated temperature CO(g)+NH3(g)⇌HCONH2(g), Kc...
Questions


History, 05.05.2020 17:41

Mathematics, 05.05.2020 17:41











Biology, 05.05.2020 17:41


Arts, 05.05.2020 17:41


Biology, 05.05.2020 17:41

Mathematics, 05.05.2020 17:41