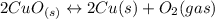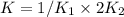Chemistry, 25.02.2020 06:14 andrewgainey1986
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1 2CuO(s) <--> Cu2O(s) + 1/2 O2(g), K2 what is K for the system 2Cu(s) + O2(g) <--> 2CuO(s) equivalent to?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1...
Questions



English, 07.12.2021 01:00


Business, 07.12.2021 01:00

Mathematics, 07.12.2021 01:00

History, 07.12.2021 01:00

English, 07.12.2021 01:00

Mathematics, 07.12.2021 01:00

Spanish, 07.12.2021 01:00


Mathematics, 07.12.2021 01:00

Chemistry, 07.12.2021 01:00



Physics, 07.12.2021 01:00


Spanish, 07.12.2021 01:00

Biology, 07.12.2021 01:00

Social Studies, 07.12.2021 01:00

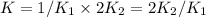
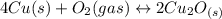
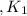 assuming equation (1)
assuming equation (1)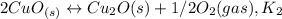 assuming equation (2)
assuming equation (2)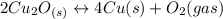 ,
,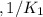 assuming equation (3)
assuming equation (3)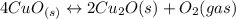
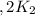 assuming equation (4)
assuming equation (4)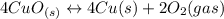
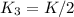 , equation (5)
, equation (5)