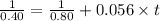Chemistry, 25.02.2020 19:44 tinajackson6534
In the reaction, A → Products, a plot of 1/[A] vs. time is linear and the slope is equal to 0.056 M−1 s−1. If the initial concentration of A is 0.80 M, how long will it take one-half of the initial amount of A to react?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1



Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
In the reaction, A → Products, a plot of 1/[A] vs. time is linear and the slope is equal to 0.056 M−...
Questions

Mathematics, 04.05.2021 21:20


Mathematics, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20


Mathematics, 04.05.2021 21:20


Mathematics, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20


Social Studies, 04.05.2021 21:20



Biology, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20


English, 04.05.2021 21:20

English, 04.05.2021 21:20

History, 04.05.2021 21:20

Mathematics, 04.05.2021 21:20

![\frac{1}{[A_t]} = \frac{1}{[A]_0}+kt](/tpl/images/0523/4978/f2ee3.png)
![[A_t]](/tpl/images/0523/4978/5262c.png) is the final concentration = Half of the initial concentration = 0.80 /2 M = 0.40 M
is the final concentration = Half of the initial concentration = 0.80 /2 M = 0.40 M![[A_0]](/tpl/images/0523/4978/9a686.png) is the initial concentration = 0.80 M
is the initial concentration = 0.80 M