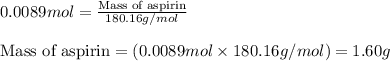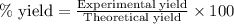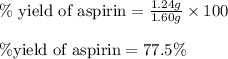Chemistry, 25.02.2020 19:53 chloegrace359
Aspirin can be made in the laboratory by reacting acetic anhydride (C 4H 6O 3) with salicylic acid (C 7H 6O 3) to form aspirin (C 9H 8O 4) and acetic acid (C 2H 4O 2). The balanced equation is
C 4H 6O 3+C 7H 6O 3?C 9H 8O 4+C 2H 4O 2
In a laboratory synthesis, a student begins with 2.80mL of acetic anhydride (density=1.08g/ml) and 1.24g of salicylic acid. Once the reaction is complete, the student collects 1.24g of aspirin.
Determine the limiting reactant for the reaction.
Determine the theoretical yield of aspirin for the reaction.
Determine the percent yield of aspirin for the reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Aspirin can be made in the laboratory by reacting acetic anhydride (C 4H 6O 3) with salicylic acid (...
Questions



History, 20.01.2020 16:31


Arts, 20.01.2020 16:31

Mathematics, 20.01.2020 16:31




Chemistry, 20.01.2020 16:31

History, 20.01.2020 16:31



Chemistry, 20.01.2020 16:31



Spanish, 20.01.2020 16:31

Social Studies, 20.01.2020 16:31


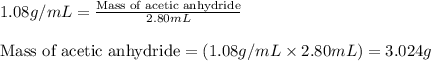
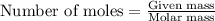 .....(1)
.....(1)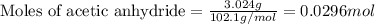
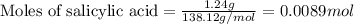
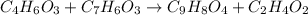
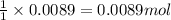 of acetic anhydride
of acetic anhydride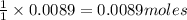 of aspirin
of aspirin