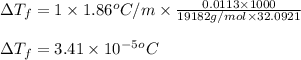A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the material in enough water to make 32.2 mL of solution. The osmotic pressure of the solution is 0.340 torr at 25°C.
(a) What is the molar mass of the gene fragment?
(b) If the solution density is 0.997 g/mL, how large is the freezing point depression for this solution (Kf of water = 1.86 °C/m)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3
You know the right answer?
A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the mat...
Questions

Mathematics, 03.05.2021 16:20


Mathematics, 03.05.2021 16:20



Chemistry, 03.05.2021 16:20


History, 03.05.2021 16:20

Mathematics, 03.05.2021 16:20



Mathematics, 03.05.2021 16:20

Mathematics, 03.05.2021 16:20

Mathematics, 03.05.2021 16:20

Mathematics, 03.05.2021 16:20

Chemistry, 03.05.2021 16:20


Arts, 03.05.2021 16:20


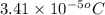
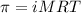
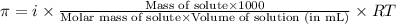
 = osmotic pressure of the solution = 0.340 torr
= osmotic pressure of the solution = 0.340 torr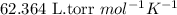
![25^oC=[273+25]=298K](/tpl/images/0523/6100/6a9f9.png)

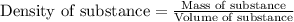
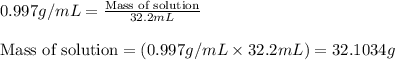
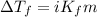
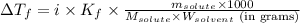
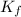 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m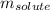 = Given mass of solute (gene fragment) = 0.0113 g
= Given mass of solute (gene fragment) = 0.0113 g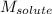 = Molar mass of solute (gene fragment) = 19182 g/mol
= Molar mass of solute (gene fragment) = 19182 g/mol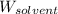 = Mass of solvent (water) = 32.0921 g
= Mass of solvent (water) = 32.0921 g