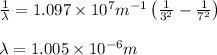Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Consider a transition of the electron in the hydrogen atom from n=3 to n=7.
a) Determine...
a) Determine...
Questions



Spanish, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31


Mathematics, 12.11.2019 04:31

English, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31

Mathematics, 12.11.2019 04:31


Spanish, 12.11.2019 04:31



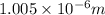
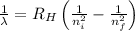
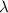 = Wavelength of radiation
= Wavelength of radiation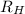 = Rydberg's Constant =
= Rydberg's Constant = 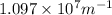
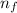 = Higher energy level = 7
= Higher energy level = 7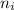 = Lower energy level = 3
= Lower energy level = 3