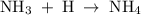Chemistry, 25.02.2020 21:09 ldpozorski
Write the equilibrium constant expression, Kc, for the following reaction: Please enter the compounds in the order given in the reaction. If either the numerator or denominator is 1, please enter 1 NH3(g) + HI(g) NH4I(s)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Write the equilibrium constant expression, Kc, for the following reaction: Please enter the compound...
Questions




Mathematics, 29.03.2021 01:10

Mathematics, 29.03.2021 01:10

History, 29.03.2021 01:10


History, 29.03.2021 01:10

Engineering, 29.03.2021 01:10

Mathematics, 29.03.2021 01:10



Social Studies, 29.03.2021 01:10

Mathematics, 29.03.2021 01:10

History, 29.03.2021 01:10

Mathematics, 29.03.2021 01:10

Chemistry, 29.03.2021 01:10


English, 29.03.2021 01:10

Mathematics, 29.03.2021 01:10

![\rm \dfrac{[NH_4]}{[NH_3]\;[H]}](/tpl/images/0523/6740/08037.png) .
.