
Chemistry, 25.02.2020 21:45 Kelshonti15
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentrations of both A and B are 2.00 mol/L. If the reaction is first order in A and first order in B, what is the rate when the initial concentration of A = 4.41 mol/L and that of B = 2.72 mol/L. Give answer to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentration...
Questions



Mathematics, 28.04.2021 21:40

Mathematics, 28.04.2021 21:40

Mathematics, 28.04.2021 21:40


Mathematics, 28.04.2021 21:40


Mathematics, 28.04.2021 21:40



Mathematics, 28.04.2021 21:40

Social Studies, 28.04.2021 21:40

Mathematics, 28.04.2021 21:40

Advanced Placement (AP), 28.04.2021 21:40

Mathematics, 28.04.2021 21:40




Mathematics, 28.04.2021 21:40

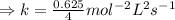
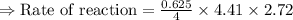
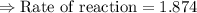 ≈1.87 mol L⁻¹s⁻¹
≈1.87 mol L⁻¹s⁻¹

