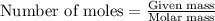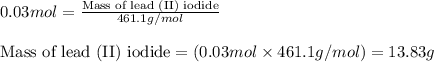Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include ph...
Questions

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00





Mathematics, 19.01.2021 14:00



Mathematics, 19.01.2021 14:00



Mathematics, 19.01.2021 14:00

Chemistry, 19.01.2021 14:00


English, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Social Studies, 19.01.2021 14:00

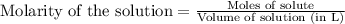
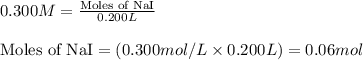
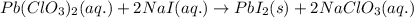
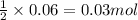 of lead (II) iodide
of lead (II) iodide