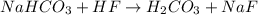Chemistry, 25.02.2020 22:06 AmyGonzalez1385
1. Acidification of sodium bicarbonate (NaHCO3) produces carbon dioxide in a two-step process. The first step produces carbonic acid (H2CO3). Write and balance the molecular equation for the first step of the reaction of sodium bicarbonate with HF.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
1. Acidification of sodium bicarbonate (NaHCO3) produces carbon dioxide in a two-step process. The f...
Questions





History, 24.08.2021 21:20




Mathematics, 24.08.2021 21:20





History, 24.08.2021 21:20

Mathematics, 24.08.2021 21:20


Mathematics, 24.08.2021 21:20



Mathematics, 24.08.2021 21:20