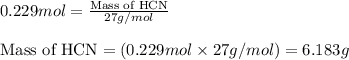Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.90 g NH3, 11.0 g O2, and 4.67 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process...
Questions

Mathematics, 18.03.2021 19:50

Chemistry, 18.03.2021 19:50


History, 18.03.2021 19:50

History, 18.03.2021 19:50



Social Studies, 18.03.2021 19:50


English, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50

Chemistry, 18.03.2021 19:50


Mathematics, 18.03.2021 19:50



Mathematics, 18.03.2021 19:50

Mathematics, 18.03.2021 19:50

Chemistry, 18.03.2021 19:50

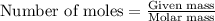 .....(1)
.....(1)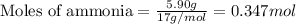
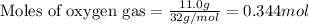
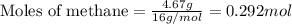
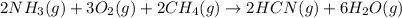
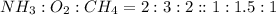
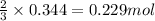 of HCN
of HCN